CDRH overview
The U.S. Food and Drug Administration’s Center for Devices and Radiological Health (CDRH) has responsibility for laser products, including proper labeling.
21 CFR 1040.10 and 1040.11 are together known as the “Federal Laser Product Performance Standard” (FLPPS).
Manufacturers self-certify to CDRH that their products comply with FLPPS requirements. This is done by submitting a Laser Product Report. CDRH will review the report and will issue an “Accession Number” which is like a file or reference number for that product.
Note that CDRH does NOT certify or approve laser products. They only review manufacturers’ self-certified reports.
If you wish to indicate your compliance with CDRH requirements, use a label such as the one shown below [note: this is coming later].
21 CFR 1040.10 and 1040.11 are together known as the “Federal Laser Product Performance Standard” (FLPPS).
Manufacturers self-certify to CDRH that their products comply with FLPPS requirements. This is done by submitting a Laser Product Report. CDRH will review the report and will issue an “Accession Number” which is like a file or reference number for that product.
Note that CDRH does NOT certify or approve laser products. They only review manufacturers’ self-certified reports.
If you wish to indicate your compliance with CDRH requirements, use a label such as the one shown below [note: this is coming later].
CDRH labels
There are two general requirements under 21 CFR 1010, which applies to all products for which an applicable standard exists:
In addition, there are three specific requirements under 21 CFR 1040.10(g) which applies to laser products:
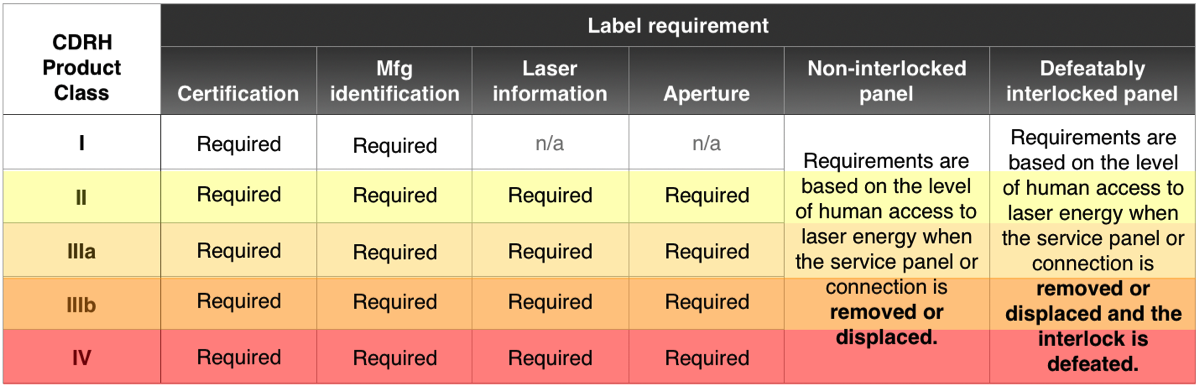
A laser product must have all five labels.
- Certification — The manufacturer certifies the product to FDA as described on this page
- Identification — Information about the manufacturer
In addition, there are three specific requirements under 21 CFR 1040.10(g) which applies to laser products:
- Laser information — Class designation, warnings and output information label
- Aperture — To show from where laser light will be emitted
- Housing panel — To indicate either non-interlocked housings, or housings that can be defeatably interlocked or where the interlock can be overridden
A laser product must have all five labels.
General CDRH label requirements
All labels provided under the FLPPS:
Note: Changes in the label requirements are provided for specific cases in FDA Notices (such as Laser Notice 50), exemptions in the standard, or upon approval of a variance.
- Must be permanently fixed or inscribed to the product
- Must be clearly visible to service their purpose and be located such that they do not require exposure to laser energy in excess of the limits associated with Class I
- Text and markings shall be legible
- The terms “Invisible” or “Visible and Invisible” shall be added before the term “Laser Radiation” when appropriate
- The term “Laser Radiation” may be substituted by the words “Laser Light” when only visible laser light is emitted
Note: Changes in the label requirements are provided for specific cases in FDA Notices (such as Laser Notice 50), exemptions in the standard, or upon approval of a variance.
Disclaimer
SAFETY NOTICE: This website is intended for the educational, instructional and informational purposes of the user and is not to be considered a substitute for a knowledgeable and trained Laser Safety Officer (LSO) with the duties and responsibilities as defined in the ANSI Z136 standard published by the American National Standard Institute.
While the labels herein may be sufficient for relatively simple and common laser types, for more complex situations, consult an expert in laser regulatory compliance.
While the labels herein may be sufficient for relatively simple and common laser types, for more complex situations, consult an expert in laser regulatory compliance.